Regulatory Procedures Search
About This Paper
Every organization is legally bound by certain rules and regulations as enacted and enforced from time to time by Government of the day.
In addition to these regulations, an organization also implements management standards like QMS / TQM / ISO-9001/ CMM etc.. This whitepaper talks about how an Artificial Intelligence powered chatbot or an enterprise search application can facilitate just-in-
time understanding of the processes and procedures or work instructions related to regulatory or quality standards like ISO 9001:2008 or ISO/TS 16949:2009 for Automotive or BIS or FSSAI or Homehealth care regulations to employees of the organization.
Introduction
An organization has huge volume of data on regulatory procedures in the form of documents and management standards like Quality manuals with work instructions for each and every department in the organization.
Most of the regulatory procedures and quality management standards is in an unstructured or semi-structured format as text or images or videos. Searching through this huge volume of unstructured data has several limitations:
Existing search methods are keyword-based, which means that they do not understand the meaning of each keyword to infer the context or
intent of the user search. On the other hand, an AI powered natural language based search can understand the intent of the user search
and shows precise results.
Users often spend more time in seeping through the results provided by keyword-based search methods.
A conversational interface that could help facilitate a clear understanding by further querying without losing the original context of
the search is too complex and nearly impossible to build when using keyword-based search methods.
And also, most of the employees in an organization should be aware of their organizational regulatory procedures and quality standards, which in other words needs considerable investments in training the employees.
Further, either the regulatory procedures are continually updated or audited at frequent intervals for compliance. In other words, there is a definitive need for employees in the organization to continuosly update their knowledge on the respective procedures or work instructions, which is a time-consuming process – not to mention the employees ability to recollect the knowledge at the hour of need.
Pharma Industry Regulations
The most important regulation that governs the production of drugs in pharma industry, especially those involved in selling their drugs in USA is Good Manufacturing Practice (GMP).
GMP regulations promulgated by the US Food and Drug Administration require that manufacturers, processors, and packagers of drugs, medical
devices take proactive steps to ensure that their products are safe, pure, and effective.
GMP regulations require a quality approach to manufacturing, enabling companies to minimize or eliminate instances of contamination, mixups, and errors. GMP regulations are to ensure a quality product to the end consumers of the drugs.
In other words, employees working in pharma industry should be trained and aware of the respective GMP procedures as mentioned in it«s best practices documentation. Further, the manufacturing processes data from each batch of products should be captured and maintained for periodic audits.
Artificial Intelligence could automate some of the above activities, like facilitating just-in-time understanding of a certian GMP procedure to an employee or sending automated alerts for possible non-compliance by parsing the huge volume of data being generated by respective manufacturing processes.
Pharmacovigilance (PV)
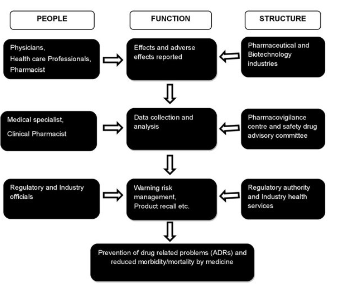
Pharmacovigilance (PV) plays a key role in the healthcare system through assessment, monitoring and discovery of interactions amongst drugs and their effects in human. PV is particularly concerned with adverse drug reactions (ADRs), which are drug responses that are noxious and unintended. The discipline of PV has developed considerably since the 1972 WHO technical report, and it remains a dynamic clinical and scientific discipline Traditionally pharma industries manually collect the information through physicians, scientists, and healthcare providers about patient’s experience and reactions on existing and new drugs. This whole information is validated through pharmacovigilance (see monitoring and reporting structure in the above sketch).
We build and host scalable, budget-friendly proxies (chatbots, virtual assistants and enterprise search applications) that can be seamlessly deployed either on the organization’s website or other content management portals or learning platforms or enterprise resource planning or human resource management systems that an organization uses internally.
We can train and deploy several proxies that can automate the complete generation, monitoring and evaluation of the data gathered as
part of the pharmacovigilance process.
Quality Management Standards (TQM / ISO 9001)
Most of the progressive organizations that are concerned with producing quality products get accredited on globally recognized quality management standards like ISO 9001 etc..
For getting ISO 9001 certification, an organization has to document and train each and every employee on the quality standards that are to be applied and implemented in their respective departments work areas.
Typically, this training is provided once in a year to the team leaders who lead the quality assurance efforts in their respective teams.
Further, the accrediting agency does an annual audit of the certification to ensure that the standards are being adhered to during the certification period.
Proxzar® can train and deploy virtual assistants that can faclitate a just-in-time understanding of a certain quality procedure or work instruction relevant to the employee«s work area / department.
Conclusion
Artificial Intelligence powered chatbots / virtual assistants / conversational agents / enterprise search applications can process large amounts of data and provide actionable insights through natural language based search.
AI technology can bring context to a user’s regulatory procedures search, notify users about the regulatory changes on time, monitor and audit organizational regulatory procedures and quality standards.
Demo of a Proxy Similar to Regulatory Procedures Search
We’ve trained a proxy that provides context based responses to Registered Nurses (RNs) in Homehealth care. RNs are mandated by law to
explain relevant topics to patients whom they visit. We’ve also seamlessly integrated the proxy into our client’s IT platform that
is used by Homehealth care agencies in USA.
For a demo of how the above proxy (which is deployed as an Enterprise Search Application) is being used to facilitate just-in-time understanding by searching unstructured data of the organization through natural language search, pl click here.
Also, the above enterprise search application can be extended by adding conversational functionality as in a chatbot.
